In practice, a metal part is immersed in a liquid media and subjected to Direct Current. The metal part is made anodic (+). The Direct Current flows from the anode to the cathode removing metal ions at a controlled rate. The amount of metal removed is dependent upon (1) the specific bath, (2) the temperature, (3) time, (4) current density, and (5) the particular alloy being electropolished.
Unlike conventional mechanical finishing systems, the electropolishing does not smear, bend, stress or fracture the crystalline metal surface to achieve smoothness and/or lustre.
Instead, electropolishing removes metal from the surface producing a unidirectional pattern that is stress-free, microscopically smooth and often highly reflective.
In addition, improved corrosion resistance and passivity are achieved on many ferrous and some non-ferrous alloys. This process is used for micro- and macro-deburring, as the reverse plating process (electropolishing) removes metal ions from the high points on the surface without etching. Burrs are removed up to 20-times faster than stock on parts proper.
Note: only limited success can be achieved with cast metals. Generally alloys containing silicon, sulfur or carbon - in appreciable amounts - will not electropolish satisfactorily.
Stainless steel investment castings are well-suited for this process.
Aluminum and zinc die castings do not electropolish well, if at all, but most other alloys of aluminum electropolish excellently. Also, 99% zinc + 1% copper electropolishes to a bright, smooth finish.
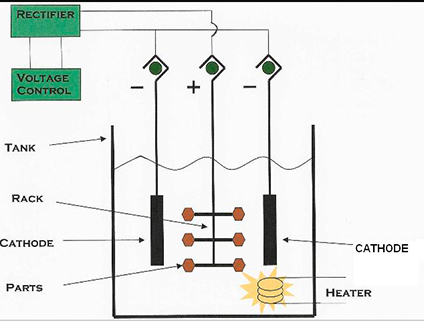
The following can be deburred and electropolished in one operation:
• Inconel, Monel, Hastelloy, Waspalloy, Compressor Blade Alloys